BrainVectors Research
News
Overview of the BrainVectors research (dowload the pdf file)
Workpackages (WP) description:
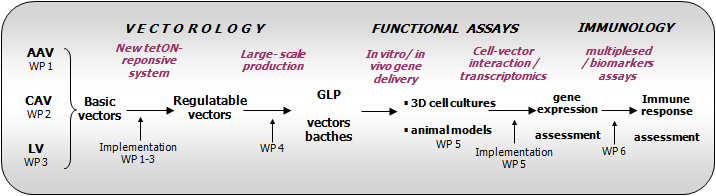
Work package 1 : Improving the Tet-On system with AAV-GDNF
|
WP leader : Liliane TENENBAUM, CHUV, Lausanne, CH |
Objectives: The goal is to derive brain-specific AAV-tetON vector incorporating tetracycline transactivator (rtTA) responding to low doses of a new clinically acceptable inducer (existing or synthesized) and eliciting no immune reaction after delivery in the brain.
The starting vector will be AAV-tetON-GDNF/2 in which the rtTA transactivator is a mutant isolated by AMC; and the tetracycline-inducible (pTet) promoter is based on a ubiquitous viral promoter (CMV). This vector responds to lower doses of dox than the first generation AAV-tetON/1-GDNF in the rat brain.
Description of work (tasks)
1.1: Bibliographic search of tetracycline derivatives or other chemically-synthesized molecules with similar structure but no antimicrobial activity passing the blood-brain barrier and, if possible, already used in clinical trials (AMC & CHUV)
1.2: In vitro evaluation of new inducers using AAV-tetON/2-GDNF (CHUV).
1.3: Report on blood & CSF concentrations of inducers administered to mice
1.4: Selection of new rtTA mutants (rtTAmut) in the presence of low doses of new inducers (evolution-driven selection) (AMC).
1.5: Predictive epitope analysis of rtTA mutants to select candidates (rtTAmut_opt) devoid of strong human epitopes (if possible) (AMC & FIRALIS).
1.6: Construct astrocyte-specific pTet promoters (FIMA) and incorporate them in AAV with improved rtTA.
Work package 2: Tet-inducible CAV-2 vectors
|
WP leader : Eric KREMER, CNRS, Montpellier, F |
|
Description of work
2.1: Generate a CAV-2 vector with reporter gene under control of Tet-regulated promoter (CAV-Tet-reporter)
2.2: Test CAV-Tet-reporter in vitro using rodent neurons and in the rodent CNS
2.3: Generate a CAV-2 vector with GDNF under control of Tet-regulated promoter (CAV-Tet-GDNF)
2.4: Test CAV-Tet-GDNF expression in vitro using rodent and in the rodent CNS
Work package 3: Development of LV vectors with Tet-On system
|
WP leader : Cecilia LUNDBERG, WNC, Lund, S |
|
Objectives The overall objectives of WP3 are to develop cell-specific LV vectors that are regulated by Tet. In more detail the objectives are:
Design, construct and validate glial-specific GFP LV vectors in vitro and in vivo
Design, construct and validate neuron-specific GFP LV vectors in vitro and in vivo
Develop GDNF expressing vectors based on the GFP vectors
Description of work
3.1 Clone the transactivators, identified in WP1, into a glia-specific LV vector plasmid under control of the GFAP promoter in a vector containing the miR 124a target site.
3.2 Clone the above into a neuron-specific LV plasmid under control of the NSE promoter in a LV vector backbone.
3.3 Construct GFP and GDNF expressing LV vectors under control of the tet-responsive promoter (TRE). TRE will be cloned upstream of either GFP or GDNF in a LV vector plasmid. These will be constructed so that they can be used in a two-vectors design or be developed into a single vector that will express the transactivator in a cell-specific fashion and the gene of interest.
3.4 Produce LV vectors of the LV vector plasmids constructed in Tasks 1-3.
3.5 Validate the expression of the vectors in the rat brain. The LV vectors will be injected into the striatum of rats. Half of the animals will be given dox in their drinking water to induce transgene expression. The transgene expression will be analyzed using IH. To evaluate cell-specificity of the vectors double IH for GFP and NeuN (neuronal marker) or GFAP (glial marker).
Work package 4: Mid-large scale vectors production
|
WP leader : Manuel CARRONDO, GENIbet, Oeiras, P |
|
Description of work For each one of the three tasks to be developed, actual middle (1L) and large scale (5 to 10L) vector production procedure will be performed. Then, for each vector, extraction of vector particles from the growth media, their purification (high resolution chromatography) and physico – biochemical characterization will be performed for quality control assays such as, homogeneity (silver-stained/ SDS-PAGE), purity (assessment of adventitious agents) and bio-safety (replication-competent wild-type virus).
4.1 Optimization of upstream/downstream vector technologies for AAV (extraction, production, purification and quality control)
4.2 Optimization of upstream/downstream vector technologies for CAV-2 (extraction, production, purification and quality control)
4.3 Optimization of upstream/downstream vector technologies for LV vectors (extraction, production, purification and quality control)
The vectors obtained in the work carried out under the three tasks above will be sent to the partners for testing in their appropriate biological tasks.
Furthermore, after the validation of different developmental stages in tasks 4.1 to 4.3, Standard Operating Procedures (SOPs) will be written according to the quality insurance standards. Such documents will be also of value if or when such vectors could require production under Good Manufacturing Practice (GMP) for futures clinical trials.
Role of GenIBET and IBET: IBET role as optimization of extraction, production, purification and GenIBET as 1 mid-scale preparations
Work package 5: Functional analysis: efficiency, specificity, cell-vector interaction
|
WP leader : Jose LANCIEGO, FIMA, Pamplona, SP |
|
WP5 analyzes the transduction efficiency, specificity, and interactions of AAV, CAV-2 and LV in 2D and 3D cultured cells and in the CNS of rodents. In addition, the effect of viral infection on cell transcriptional pattern will be analyzed.
Description of work
5.1. Classic primary cultures of human midbrain neurons and glia will be used to analyse vector-cell interaction. Vector transduction efficiency and specificity will be analysed by FACS, confocal microscopy and immunocytochemistry, using antibodies against the transgene and cell specific antibodies (UniRM).
5.2. Transduction efficiency and specificity will be analysed in 3D suspension cultures developed by IBET. 3D suspension cultures are composed by neurons and glia that form spheres and tight cell-cell interactions. 3D cultures will be infected with the different virus and vector cell interaction, transduction efficiency and specificity will be analysed as described in Task 5.1. (UniRM & IBET).
5.3. Transduction efficiency and specificity will be analysed in vivo after sterotaxic injection of the viruses into the brain of humanized mice (CHUV) and standard rats. In mice, bilateral dopaminergic depletion will be induced by the chronic intraperitoneal delivery of the specific dopaminergic neurotoxin MPTP (CHUV). In rats, a unilateral nigrostriatal lesion will be induced following the stereotaxic intracerebral delivery into the medial forebrain bundle of the dopaminergic neurotoxin 6-OHDA (FIMA). Unilateral viral vector injections will be performed and the contralateral tissue will be used as control. Transduction efficiency and specificity will be analysed by immunohistochemistry. Furthermore GDNF efficacy will be analyzed by behavioural assessment, histological post-mortem examination (densitometry of TH-immunopositive fibers and terminals in the striatum) as well as by quantification of the dopaminergic cells in substantia nigra using sterology (FIMA & CHUV).
5.4: Neuroprotection and behavioural tests in MPTP mice and 6-OHDA rats by by optimized AAV-tetbrainON:inducers pairs as compared to initial AAV-tetON/2-GDNF (CHUV). Neuroprotection will be assessed by histological staining and stereological counts of dopaminergic neurons in the substantia nigra in control (uninjected), lesioned untreated and lesioned GDNF-treated animals. Behavioral abnormalities induced by the PD models as well as GDNF-induced recovery will be assessed by typical motor tests (amphetamine-induced rotations, rotarod, forelimb asymmetry). (CHUV). At different stages of vector development, AAV-tetON-GDNF (AAV-tetON2; AAV-tetbrainON- rtTAmut_opt- GDNF) will be injected in MPTP-treated, immunologically humanized mice (provided by FIRALIS). AAV serotype will be selected according to the results of WP6. Blood samples will be sent to FIRALIS for immunological analysis at different times post-injection (see WP6)
5.5 Transcriptional response due to vector infection will be performed following incubation with cultures of brain cells by transcriptome analysis. Total RNA will be collected and used to generate double-stranded cDNA using standard procedures. cRNAs will be synthesized, cleaned and fragmented using Affymetrix kits. All biotinylated cRNA samples will be quality checked on a test-chip and RNA samples passing the test-chip quality control will be further analyzed. Arrays will be scanned on an Affymetrix scanner. At least two experimental aspects are crucial to optimize information from microarray studies: a defined experimental design and an extensive elaboration of data. For the first aspect, we will study the temporal response to distinguish between an acute and/or chronic/delayed response. Analysis of the arrays will be performed as previously described. For the comparative analysis of gene expression variations at each time point, we will compare mock-treated control arrays vs. vector-treated arrays. Our data will be analyzed for fold-change variations and functionally clustered. Pathway and promoter analysis of the regulated genes will also be studied. Our principle validation techniques will be qRT-PCR and Luminex assays for mRNA and protein quantification.
Data analysis: Data collected will be analyzed adhering to the MIAME guidelines and using the MIAME checklist. For the relative analysis of gene expression variations at each time point, we will compare mock-treated control arrays vs. vector-treated arrays. (UniRM).
Work package 6: Analysis of the immune responses
|
WP leader : Hueseyin FIRAT, FIRALIS, Huninge, F |
|
Objectives
WP6 aims to investigate the molecular basis of the immune reaction derived by CD8 T cells against vectors and transgenes and obtain specific and sensitive translational biomarkers in animal models. The ultimate usage of these biomarkers will be used for the monitoring of the immune events that occurs in patients undergoing gene therapy trials related to Parkinson’s disease.
Description of work
6.1.Identification of CD8 epitopic peptides derived from vector constituents and transgenes using epitopic motif search programs, in vitro binding assays. FIRALIS will perform (in silico) analysis on sequences of the recombinant vectors and transgenes to identify MHC-restricted epitopes.
6.2. In vivo assessment of the candidate epitopic peptides from vectors and transgenes in "HLA-humanized" mice. The HLA-restricted immunogenic capacity of each peptide will be compared to select the immune-dominant epitopes for selected HLA haplotypes e.g., HLA-A2.1.
6.3 Implementation of the Parkinson’s disease model in the “HLA- humanized mice” model. The HLA-humanized mice with the appropriate genetic background will be treated with MPTP to induce stable behavioral changes typical of parkinsonism.
6.4 Potential HLA-responses in MPTP model of HLA-humanized mice (e.g., HLA-A02.01/HLA-DR1 and human CD8/CD4-transgenic, H-2 class I/class II knockout mice ) will be used to elucidate diverse immune responses (human CD8, CD4-restricted CTL and antibody responses) against the AAV-, CAV- and LV-tetON-M2-empty vectors. These vectors will be injected into the striatum of mice at several different time points starting from 10 days until 3 months of age. In parallel, neutralizing circulating antibodies will be measured.
6.5 In vivo evaluation of the immunological properties of recombinant vector constructs in correlation with their efficacy, toxicity, and pharmacological properties against the viral capsid and the rtTA transactivator.

